Only for Licensed Professionals
Only for Licensed Professionals
.webp)
Mounjaro FDA Approval Status
David Fuller
Last Updated On: October 24, 2025
The U.S. Food and Drug Administration (FDA) plays a critical role in evaluating and approving new treatments that can improve public health. Every year, the FDA rigorously reviews therapies for safety, effectiveness, and overall quality—particularly those designed to manage chronic conditions like diabetes and obesity, which are among the most common and challenging health issues worldwide.
One of the most significant recent FDA approvals is Mounjaro (tirzepatide), which received authorization in May 2022 for the treatment of type 2 diabetes in adults. While the approval was focused on improving blood sugar control, Mounjaro’s impressive results in weight loss during clinical trials sparked considerable interest. This led to another breakthrough in November 2023, when the FDA approved tirzepatide under the brand name Zepbound for chronic weight management.
In this article, we’ll explore Mounjaro’s FDA approval status, the approved indications, and the exciting ongoing studies that may open doors to new potential uses for this promising therapy.
Key Takeaways
- Mounjaro (tirzepatide) is FDA-approved for type 2 diabetes and, under the brand name Zepbound, for weight management.
- Dual receptor action on GIP and GLP-1 hormones improves insulin sensitivity, reduces appetite, and supports weight loss.
- SURPASS clinical trials showed significant improvements in HbA1c and body weight for patients with type 2 diabetes.
- Mounjaro is effective as a second-line therapy after metformin and works well alongside other antidiabetic treatments.
- FDA approval of Zepbound expanded Mounjaro’s use to include chronic weight management, marking a breakthrough in treating obesity with metabolic benefits.
- Routine monitoring is necessary for gastrointestinal effects and thyroid risks, as noted in the FDA prescribing guidelines.
About: Operating since 2016, Med Supply Solutions is known for being one of the industry’s top and trusted suppliers of cosmetic and viscosupplementation products. If you’re looking to buy Mounjaro online, contact our sales department for more information.

The Clinical Trials That Led to Mounjaro’s FDA Approval

The FDA approval of Mounjaro (tirzepatide) was driven by the results of the SURPASS clinical trial series, which involved over 5,000 adults with type 2 diabetes. These trials compared tirzepatide to placebo, insulin glargine, and semaglutide, evaluating its ability to manage blood sugar levels and support weight loss.
Key Findings
- HbA1c reduction: Up to 2.4% (with the 15 mg dose) demonstrated in the SURPASS trials, which was generally superior to active comparators, exceeding most existing non-insulin therapies.
- Weight loss: Patients experienced an average weight loss of 12–25 pounds, depending on the dose and baseline weight.
- Improved glucose control: Significant reductions in fasting glucose and post-meal glucose levels.
- Dual action mechanism: The GIP and GLP-1 receptors activated by tirzepatide work together to regulate glucose more effectively than traditional single-receptor treatments.
These trial results were pivotal in securing Mounjaro’s approval. The unique dual-action of tirzepatide that can target both GIP and GLP-1 makes it an innovative treatment option, offering enhanced metabolic control, weight loss, and improved insulin sensitivity.
How FDA Approval Defines Mounjaro’s Use in Diabetes Management
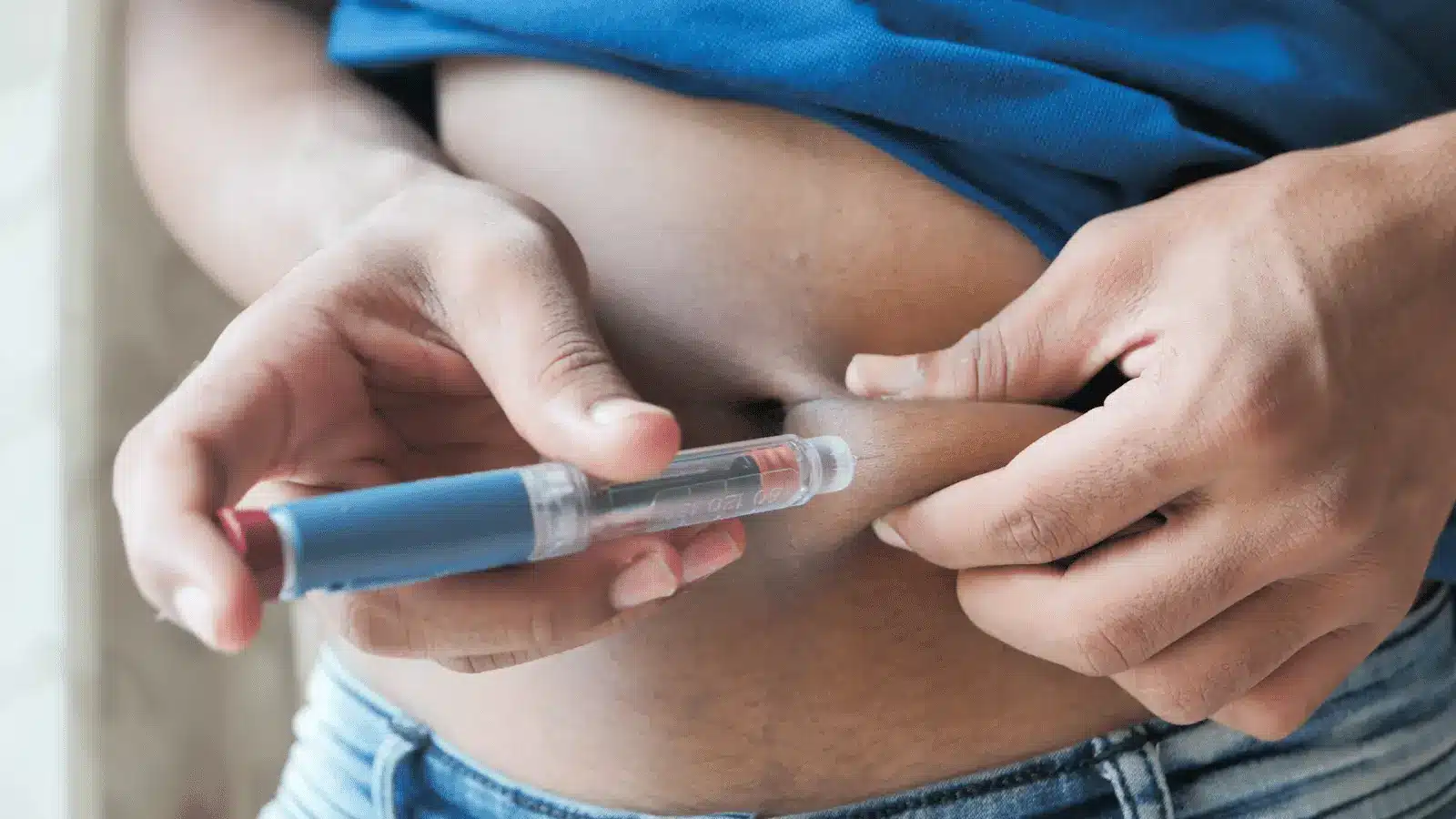
The FDA approval of Mounjaro is for adults with type 2 diabetes mellitus (T2DM) to improve blood sugar control as part of a comprehensive approach that includes diet and exercise. It is not approved for use in type 1 diabetes or diabetic ketoacidosis.
Prescribing Guidelines
- Starting dose: Mounjaro begins at 2.5 mg weekly, gradually increasing over time.
- Titration: The dose is typically escalated every 4 weeks to improve tolerance and minimize gastrointestinal side effects.
- Second-line therapy: It’s often used after metformin or in combination with other antidiabetic agents, aligning with major clinical guidelines.
Mounjaro’s approval positions it as a valuable addition to diabetes care. It offers superior glycemic control compared to many other therapies, including insulin and GLP-1 receptor agonists. Beyond controlling blood sugar, Mounjaro has demonstrated weight control benefits and improved cardiovascular risk factors, such as blood pressure and lipid levels.
Clinical trial data also indicates a reduction in major adverse cardiovascular events (MACE). This further aligns Mounjaro with modern diabetes care goals focused on comprehensive metabolic health.
The Expansion of Mounjaro Approval for Weight Management as Zepbound
In November 2023, the FDA expanded the approval of tirzepatide under the brand name Zepbound. This treatment isWWWWD for chronic weight management in adults with obesity (BMI ≥30) or overweight (BMI ≥27) with at least one related comorbidity, such as type 2 diabetes, hypertension, or dyslipidemia.
This approval marked a significant shift in how we approach weight management, acknowledging obesity as a treatable chronic disease. With Zepbound, tirzepatide provides a comprehensive metabolic solution—targeting both weight loss and glycemic control simultaneously.
Distinctions between Mounjaro and Zepbound
- Mounjaro focuses on glycemic control in type 2 diabetes through dual incretin action.
- Zepbound delivers sustained weight loss and cardiometabolic improvement, targeting those with obesity or overweight and associated conditions.
- Shared active ingredient: Both medications contain tirzepatide. However, their dosing, endpoints, and clinical goals vary slightly to address each condition’s unique needs.
- Dosing flexibility: Zepbound follows a similar structured titration schedule. However, HCPs tailor it for weight outcomes rather than blood sugar regulation.
The expansion of Mounjaro to include Zepbound underscores the growing recognition of obesity as a chronic condition that requires long-term pharmacological management. By providing an evidence-based approach to weight loss and metabolic health, tirzepatide is bridging diabetes and obesity with one effective treatment.
What FDA Approval of Mounjaro Means for Patients and Clinicians
Mounjaro’s FDA approval opens the door to a new era of metabolic management for patients. It not only offers enhanced blood sugar control but also provides significant weight loss benefits for individuals with type 2 diabetes or obesity. For clinicians, it offers a flexible therapeutic tool to treat complex metabolic disorders with a once-weekly injection.
However, patients must be mindful of potential interactions when combining Mounjaro with other medications. For example, if you’re taking antibiotics with Mounjaro, despite there being no major interactions, the delayed gastric emptying caused by Mounjaro may slightly affect how quickly some antibiotics are absorbed.
Benefits of Mounjaro
- Dual receptor mechanism: Provides improved insulin sensitivity and appetite regulation.
- Cardiovascular benefits: Long-term studies, such as the SURPASS-CVOT and SUMMIT trials, show favorable outcomes in reducing cardiovascular risks.
- Once-weekly dosing: Designed to improve patient adherence, making it easier for patients to stick to their treatment plan.
While Mounjaro’s benefits are substantial, clinicians must remain mindful of potential contraindications and side effects. Individuals with a personal or family history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) should exercise extra caution. Routine monitoring and careful adherence to FDA guidelines are essential to ensure safe and effective use.
Conclusion
Mounjaro’s journey from research to FDA approval is a milestone in metabolic medicine. As the first dual incretin agonist approved for type 2 diabetes, and now for weight management under Zepbound, tirzepatide offers patients an innovative approach to managing glucose levels and body weight simultaneously.
Its robust clinical data, unique mechanism of action, and favorable safety profile make Mounjaro an exciting option for those managing diabetes and obesity. As ongoing studies explore its broader benefits, Mounjaro stands at the forefront of modern chronic disease management.
FAQs
1. When did the FDA approve Mounjaro?
The FDA approved Mounjaro (tirzepatide) in 2022. It is for improving blood sugar control in adults with type 2 diabetes.
2. Does Mounjaro have FDA approval for weight loss?
Yes. Tirzepatide received separate FDA approval under the brand name Zepbound in November 2023 for chronic weight management.
3. What is the difference between Mounjaro and Zepbound?
Both contain tirzepatide. Mounjaro focuses on diabetes management, while Zepbound targets obesity and related conditions.
4. Is Mounjaro safe for long-term use?
Long-term studies suggest a favorable safety profile. However, healthcare professionals should regularly monitor their patients for gastrointestinal effects and thyroid-related risks.
References
Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021;385(6):503-515. doi:10.1056/NEJMoa2107519
Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398(10313):1811-1824. doi:10.1016/S0140-6736(21)02188-7
Tirzepatide injection. Cleveland Clinic. https://my.clevelandclinic.org/health/drugs/23789-tirzepatide-injection
Products
Cart
Log In
Newsletter
Subscribe for exclusive offers and updates on new arrivals
Share feedback at:
Working Hours
MON - SUN 9AM to 6PM EST
The Most Popular Brands
Med Supply Solutions
Support
Secure checkout is guaranteed with full adherence to PCI DSS payment standards.
Products listed here are guaranteed authentic and manufacturer-sourced.
Pay easily with trusted providers


*Google and Apple Pay are currently only available via a direct link provided by your account manager.
Copyright 2026. Med Supply Solutions