Monovisc FDA Approval – Is It Safe to Use?
David Fuller
Last Updated On: October 2, 2024
In 1997, the Food and Drug Administration (FDA) approved viscosupplementation as a conservative treatment for osteoarthritis (OA), significantly advancing in managing this chronic condition. Since then, viscosupplements like hyaluronic acid injections have become popular for alleviating joint pain and improving mobility in OA patients.
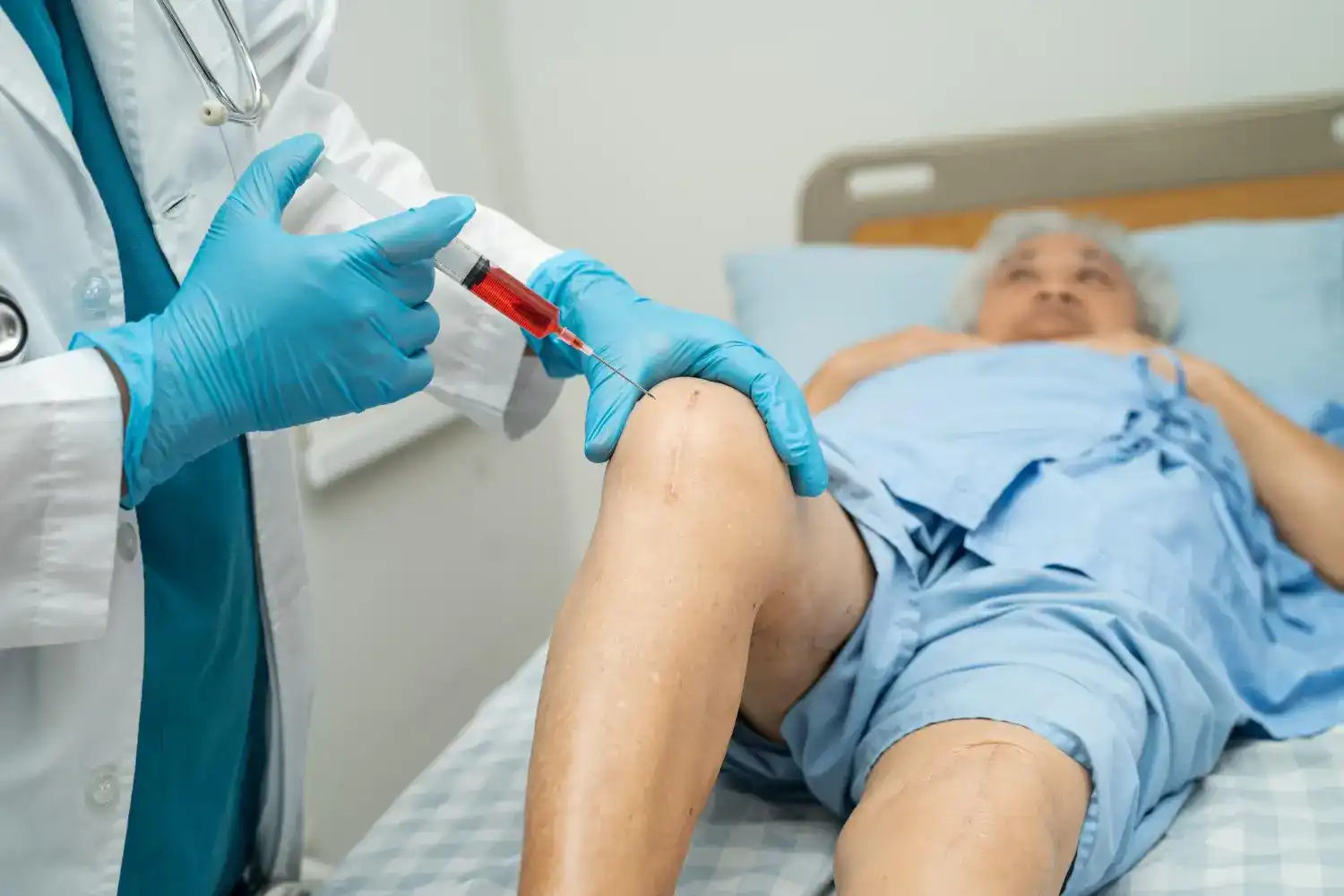
Monovisc is a single-injection treatment designed to provide long-lasting relief from knee OA pain by restoring the natural lubrication and cushioning of the joint. Understanding the safety and efficacy of Monovisc is essential for those considering it as a treatment option.
In this article, we will explore the FDA approval process for Monovisc, examine its safety profile, and discuss what patients can expect from this treatment.
Key Takeaways
- Monovisc is an FDA-approved single-injection treatment for knee osteoarthritis (OA). Its approval signifies that it has undergone rigorous testing to demonstrate safety and efficacy.
- Monovisc contains non-animal-sourced hyaluronic acid, which closely mimics the body’s natural joint fluid. It provides lubrication and cushioning to reduce pain and improve mobility.
- It offers quick relief from knee pain and lasting effects for several months, reducing the need for frequent follow-up treatments.
- The single-injection regimen simplifies treatment, making it more convenient than multi-session viscosupplementation options.
About: Operating since 2016, Med Supply Solutions is known for being one of the industry’s top and trusted suppliers of cosmetic and viscosupplementation products. If you’re looking to buy Monovisc online, contact our sales department for more information.
MONOVISC®
SODIUM HYALURONATE
$349.00
Tier pricing
Save 2.87%
3 or more
$339.00 each
Save 4.3%
11 or more
$334.00 each
Save 5.73%
21 or more
$329.00 each
Understanding Monovisc and FDA Approval
Monovisc is a single-injection treatment designed to alleviate knee pain caused by osteoarthritis. It is formulated with hyaluronic acid derived from non-animal sources, making it suitable for a wide range of patients, particularly those who haven’t found relief with other arthritis treatments.

- Composition: Monovisc contains non-animal-sourced hyaluronic acid, which closely resembles the body’s natural joint fluid, providing lubrication and cushioning to reduce pain and improve mobility.
- Usage: This treatment is ideal for patients with knee osteoarthritis who have not experienced satisfactory results with other medications or therapies. Monovisc’s single-injection regimen offers the convenience of significant relief without the need for multiple visits.
- FDA Approval: The FDA has approved Monovisc following a thorough evaluation process that requires substantial evidence of its safety and efficacy compared to existing treatment options. The FDA’s approval signifies that Monovisc meets high standards for effectiveness, making it a trusted choice for knee pain management.
- Convenience: Patients can achieve significant relief with one injection, making Monovisc an efficient and practical treatment option for managing osteoarthritis pain.
It is important to note that patients should avoid Monovisc if they are allergic to hyaluronate preparations. Thanks to the FDA’s rigorous approval process, treatments like Monovisc offer a safe and effective option for knee pain management.
The convenience of a single injection and its long-lasting effects make Monovisc a preferred choice, especially when compared to alternatives like Monovisc vs Durolane.
Safety Data and Clinical Trials
Monovisc received FDA approval based on a pivotal phase III clinical trial. This randomized, controlled, double-blind, multicenter study involved 369 patients with osteoarthritis (OA) of the knee across 31 centers in the US and Canada. The study aimed to evaluate the safety and effectiveness of Monovisc for treating joint pain.

Another significant study was conducted at 31 sites across the US between January 2008 and December 2009. This trial examined the safety and effectiveness of Monovisc as a single intra-articular hyaluronic acid (HA) injection for treating idiopathic, symptomatic knee OA.
Anika Therapeutics’ white paper reviews data from published pivotal clinical studies and post-marketing safety databases. It compares Monovisc to other HA treatments like DUROLANE®, Gel-One®, and Synvisc-One®. The analysis suggests that Monovisc provides early and durable pain relief in a single high-dose injection, making it a convenient and effective option for patients.
These studies highlight Monovisc’s potential as a safe and effective treatment for knee osteoarthritis, offering durable pain relief with a single injection.
Implications for Clinical Use
Monovisc offers new options for treating knee pain caused by osteoarthritis, offering significant benefits for patients and healthcare providers. However, weighing the benefits and considerations before selecting this treatment is crucial.

- Single Injection: Monovisc is the first FDA-approved single-injection treatment using non-animal-sourced hyaluronic acid, making it a convenient choice compared to therapies requiring multiple injections.
- Non-Animal Source: The hyaluronic acid in Monovisc comes from non-animal sources, reducing the risk of allergic reactions and making it safer for sensitive individuals.
- FDA Approval: Having undergone rigorous FDA evaluation, Monovisc meets strict standards for safety and efficacy, providing patients and healthcare providers with added confidence.
- Suitable for Resistant Cases: Monovisc is designed for patients who have not found relief from other treatments, such as exercise or oral pain medications, offering a new path to symptom management.
- Quick Relief and Long-Lasting Results: Many patients experience rapid relief from knee pain following the injection, with effects lasting for several months, minimizing the need for frequent follow-up visits.
- Reduced Side Effects: Compared to other treatments, Monovisc typically results in fewer side effects, contributing to its reputation as a safe and effective option.
- Sterile and High-Quality: Monovisc is produced to high safety and purity standards, ensuring that each injection is sterile and clear for maximum patient safety.
Monovisc offers hope for those with knee osteoarthritis, particularly those who have tried other methods without success. It provides an effective solution that balances convenience with long-term benefits.
Considerations for Patients and Healthcare Providers
Before administering Monovisc, healthcare providers must evaluate patients for potential allergies to hyaluronate products to ensure the treatment is safe. Providers must also follow the recommended dosage and monitor patients closely for any side effects following the injection.
Patients should openly discuss their medical history and any concerns with their healthcare provider to ensure that Monovisc is the right treatment for them. With its FDA approval and well-established safety profile, Monovisc can effectively manage knee osteoarthritis, but individual considerations are crucial for achieving the best outcomes.
Conclusion
Monovisc has received FDA approval, signifying that it has undergone rigorous testing to demonstrate its safety and efficacy for treating knee pain associated with arthritis. Before administering Monovisc, healthcare providers assess whether other treatments have been insufficient. This approval also offers patients a viable option free from animal-derived components. Therefore, Monovisc is a safe choice for those requiring effective knee pain management.
FAQs
1. What is Monovisc, and how does it work?
Monovisc is a single-injection treatment for knee osteoarthritis that uses hyaluronic acid derived from non-animal sources. It works by restoring lubrication and cushioning in the joint, reducing pain, and improving joint function.
2. How long does Monovisc provide relief for knee osteoarthritis?
Monovisc typically provides relief for up to six months after a single injection, minimizing the need for multiple treatment sessions.
3. What are the common side effects of Monovisc?
Common side effects include mild pain, swelling, or redness at the injection site. These symptoms typically resolve on their own within a few days. If symptoms persist, consult your healthcare provider for further guidance.
4. Who should avoid using Monovisc?
Patients who are allergic to hyaluronate or have had adverse reactions to similar products should avoid Monovisc.
References
Viscosupplementation in the Therapy for Osteoarthritic Knee. Appl Sci. 2021;11(24):11621. doi:10.3390/app112411621.
Monovisc (sodium hyaluronate) FDA Approval History. Drugs.com. https://www.drugs.com/history/monovisc.html
Products
Cart
Log In
Newsletter
Subscribe for exclusive offers and updates on new arrivals
Share feedback at:
Working Hours
MON - SUN 9AM to 6PM EST
The Most Popular Brands
Med Supply Solutions
Support
Secure checkout is guaranteed with full adherence to PCI DSS payment standards.
Products listed here are guaranteed authentic and manufacturer-sourced.
Pay easily with trusted providers


Copyright 2025. Med Supply Solutions